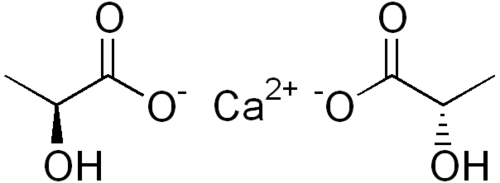Calcium Compound
Manufacturer, supplier, trader, dealer exporter of Calcium Compound in Mumbai India, Dubai, Qatar, Yemen, Saudi, South Africa, Lima Peru, Netherlands, New Zealand, Australia and many more. Our product range includes a wide range of calcium phosphate tri, calcium chloride dihydrate, calcium acetate, calcium carbonate, calcium chloride fused and calcium gluconate.
Calcium Acetate
Owing to our years of industry experience, we are offering a premium grade of Calcium Acetate. The offered calcium acetate is used to prevent high blood phosphate levels in patients who are on dialysis due to severe kidney disease. As well, this calcium acetate is well tested on assorted parameters in order to offer a pure range to our clients’. This calcium acetate is available in variegated packaging options as per customers’ demands.
Features:
- Highly effective
- Used as a food additive
- Control phosphate level
Calcium Acetate Dried
Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime.
Canada Balsam (8007-47-4)
| Specifications | |
|---|---|
| Appearance | Clear colorless liquid. |
| Natural drying time | 2-3 days |
| Refractive index (15°C; 589 nm) | 1.525-1.535 |
| Oven drying time (at 60-80°C) | 1 hour |
Calcium Carbonate
Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks in all parts of the world, and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime, and is created when Ca ions in hard water react with carbonate ions creating limescale. It is commonly used medicinally as a calcium supplement or as an antacid, but excessive consumption can be hazardous.
Calcium Chloride Dihydrate
Calcium Chloride Dihydrate is an excellent water soluble crystalline Calcium source for uses compatible with chlorides. Chloride compounds can conduct electricity when fused or dissolved in water. Chloride materials can be decomposed by electrolysis to chlorine gas and the metal. They are formed through various chlorination processes whereby at least one chlorine anion (Cl-) is covalently bonded to the relevant metal or cation. Ultra high purity and proprietary formulations can be prepared.
Calcium Chloride Fused
Calcium Chloride Fused, CaCl2, is a salt of calcium and chlorine. It behaves as a typical ionic halide, and is solid at room temperature. Common applications include brine for refrigeration plants, ice and dust control on roads, and desiccation. Because of its hygroscopic nature, anhydrous calcium chloride must be kept in tightly sealed, air-tight containers.
Calcium Citrate Tribasic Tetrahydrate
Being one of the renowned organizations in the market, we are highly engaged in offering Calcium Citrate Tribasic Tetrahydrate. The offered product is processed utilizing best in class chemical compounds and modern technology in line with the industry standards. Our given product is a high-quality calcium source for food fortification. Our valuable customers can avail this product at market price.
Features:
- Sour in taste
- Good solubility at low PH.
- Accurate composition
Calcium Formate
Calcium formate, Ca(HCOO)2, is the calcium salt of formic acid, HCOOH. It is also known as food additive E238 in food industry. The mineral form is very rare and called formicaite. It is known from a few boron deposits. It may be produced synthetically by reacting calcium oxide or calcium hydroxide with formic acid.
Calcium Fluoride
Calcium fluoride is the inorganic compound with the formula CaF2. It is a colourless insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.
Calcium Gluconate
Calcium gluconate is a mineral supplement. It is manufactured by the neutralization of gluconic acid with lime or calcium carbonate.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.
Calcium Hydroxide
A wide quality array of products comprising Chemical Compound, Organic Compound, Boric Acid, Carnauba Wax, Crude Iodine, Ammonium Ferric Citrate, Gelatinous Substance, Inorganic Compound, Immersion Oil, Laboratory Chemicals,Magnesium Metal Turning, Wool Wax, Acenaphthene Chemical, Magnesium Metal Powder and Malt Sugar.
Calcium Hydrogen Phosphate Dihydrate
| Specifications | |
|---|---|
| Appearance | White crystalline powder |
| Assay (complexometric) | 98 - 105% |
| Substance insoluble in HCl | Max 0.2% |
| Calcium hydrogen and tri-calcium phosphate | Passes test |
| Carbonate (as CO3) | Passes test |
| Chloride (Cl) | Max 0.125% |
| Fluoride (F) | Max 0.005% |
| Sulphate (SO4) | Max 0.5% |
| Heavy metal (as Pb) | Max 0.003% |
| Arsenic (As) | Max 0.0001% |
| Barium (Ba) | Passes test |
| Iron (Fe) | Max 0.04% |
| Loss on ignition (at 800°C) | 24.5 - 26.5% |
Calcium Iodide
Calcium iodide is the inorganic compound with the formula CaI2. This colourless deliquescent solid is highly soluble in water. Its properties are similar to those for related salts, such as calcium chloride. It is used in photography.It's also used in cat food as a source of iodine.
Calcium Lactate
Calcium lactate is a black or white crystalline salt made by the action of lactic acid on calcium carbonate. It is used in foods (as an ingredient in baking powder) and given medicinally. Its E number is E327. It is created by the reaction of lactic acid with calcium carbonate or calcium hydroxide.Cheese crystals usually consist of calcium lactate, especially those found on the outside, on younger cheese, and on Cheddar cheese. In medicine, calcium lactate is most commonly used as an antacid and also to treat calcium deficiencies. Calcium lactate can be absorbed at various pHs and does not need to be taken with food for absorption for these reasons.Calcium lactate is added to sugar-free foods to prevent tooth decay. When added to chewing gum containing xylitol, it increases the remineralization of tooth enamel.[3] It is also added to fresh-cut fruits such as cantaloupes to keep them firm and extend their shelf life, without the bitter taste caused by calcium chloride, which can also be used for this purpose.
Calcium Nitrate Tetrahydrate
Calcium nitrate, also called Norgessalpeter (Norwegian saltpeter), is the inorganic compound with the formula Ca(NO3)2. This colourless salt absorbs moisture from the air and is commonly found as a tetrahydrate. It is mainly used as a component in fertilizers but has other applications. Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. A variety of related salts are known including calcium ammonium nitrate decahydrate and calcium potassium nitrate decahydrate.
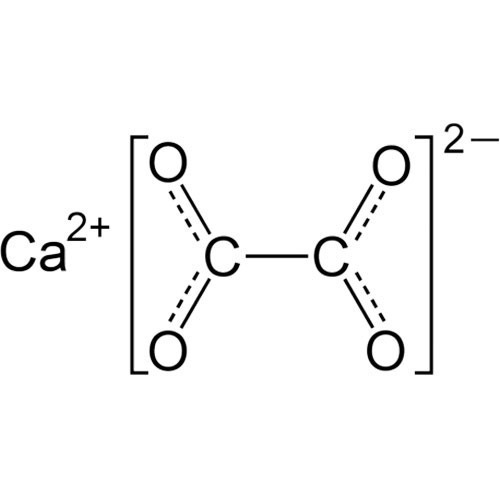
Calcium Oxalate
Calcium oxalate (in archaic terminology, oxalate of lime) is a chemical compound that forms envelope-shaped crystals, known in plants as raphides. A major constituent of human kidney stones, the chemical is also found in beerstone, a scale that forms on containers used in breweries. Its chemical formula is CaC2O4 or Ca (COO)2.
Calcium Oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "lime" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, "quicklime" specifically applies to the single chemical compound calcium oxide. Calcium oxide which survives processing without reacting in building products such as cement is called free lime.
Calcium Propionate
Calcium propanoate or calcium propionate has the formula Ca(C2H5COO)2. It is the calcium salt of propanoic acid.
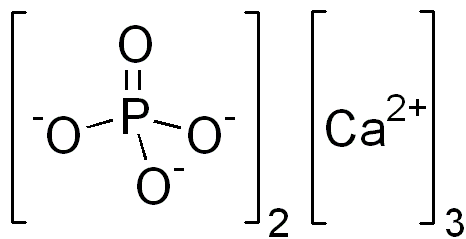
Calcium Phosphate Tri
Tricalcium phosphate (sometimes abbreviated TCP) is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). Calcium phosphate is one of the main combustion products of bone (see bone ash). Calcium phosphate is also commonly derived from inorganic sources such as mineral rock.
Calcium Stearate
We are engaged in providing a top class Calcium Stearate..
Features:
- Longer shelf life
- Highly effective
- Precise composition
- Safe to use
- Accurate results
- Reliable nature
We are offering wide range of fine and heavy chemical:
- Zinc Stearate (Liquid)
- Zinc Oxide
- Zincox Active Grade
- Zinc Carbonate
- Calcium Stearate SPC Brand
- Zinc Chloride
- Zinc Sulphate
- Zinc Sulphate (Brooksite)
Calcium Sulphate Anhydrous
Calcium sulfate (or calcium sulphate) is a common laboratory and industrial chemical. In the form of γ-anhydrite (the nearly anhydrous form), it is used as a desiccant. It is also used as a coagulant in products like tofu.In the natural state, unrefined calcium sulfate is a translucent, crystalline white rock. When sold as a color-indicating variant under the name Drierite, it appears blue or pink due to impregnation with Cobalt(II) chloride, which functions as a moisture indicator. The hemihydrate (CaSO4·~0.5H2O) is better known as plaster of Paris, while the dihydrate (CaSO4·2H2O) occurs naturally as gypsum. The anhydrous form occurs naturally as β-anhydrite. Depending on the method of calcination of calcium sulfate dihydrate, specific hemihydrates are sometimes distinguished: alpha-hemihydrate and beta-hemihydrate.They appear to differ only in crystal shape. Alpha-hemihydrate crystals are more prismatic than beta-hemihydrate crystals and, when mixed with water, form a much stronger and harder superstructure.
Calcium Sulphate Dihydrate
Calcium sulfate (or calcium sulphate) is a common laboratory and industrial chemical. In the form of γ-anhydrite (the nearly anhydrous form), it is used as a desiccant. It is also used as a coagulant in products like tofu.In the natural state, unrefined calcium sulfate is a translucent, crystalline white rock. When sold as a color-indicating variant under the name Drierite, it appears blue or pink due to impregnation with Cobalt(II) chloride, which functions as a moisture indicator. The hemihydrate (CaSO4·~0.5H2O) is better known as plaster of Paris, while the dihydrate (CaSO4·2H2O) occurs naturally as gypsum. The anhydrous form occurs naturally as β-anhydrite. Depending on the method of calcination of calcium sulfate dihydrate, specific hemihydrates are sometimes distinguished: alpha-hemihydrate and beta-hemihydrate.They appear to differ only in crystal shape. Alpha-hemihydrate crystals are more prismatic than beta-hemihydrate crystals and, when mixed with water, form a much stronger and harder superstructure.
Chemical Products
- Aluminium Compound
- Ammonium Compound
- Antimony Compound
- Barium Compound
- Bismuth Compound
- Boric Acid
- Cadmium Compound
- Calcium Compound
- Cetramide
- Chemical Compound
- Chemical Element
- Copper Compound
- Cuprous Compound
- Ferric Compound
- Ferrous Compound
- Food Additives
- Gelatinous Substance
- Gum Powder
- Inorganic Compound
- Laboratory Chemicals
- Laboratory Equipment
- Lead Compound
- Lithium Compound
- Manganese Compound
- Magnesium Compound
- Magnesium Metal Turning
- Mercuric Compound
- Micrcoscopy Stains And Solutions
- Natural Oil
- Nickel Compound
- Organic Compound
- Potassium Compound
- Speciality Chemicals
- Silver Compound
- Sodium Part I
- Sodium Part II
- Sodium Part III
- Strontium Compound
- Zinc Compound